Smell and the Taste:
In our life we face many questions, I will start with the spoiled tomato, it will produce some bad essence. If we start to ask question with our roommates or friends or in family.
Will the smell remains same for all the noses or it varies?
When I started to search on this topic, I got many information. For answer you need to spend lot of time to read this full article.
If I go to any shopping mall by closing my eyes, I can able to tell thousand of materials by smelling it. Second question rises to mind, Like how many my nose can detect.
It is tough biology ? For this easy thinking three people got Noble prize for medicine in 2004.
If you have any doubts, comment on this full article. Take your time to read the full article.
SMELL AND MEMORY
By Shigeyuki Ito
"When nothing else subsists from
the past, after the people are dead, after the things are broken and scattered·
the smell and taste of things remain poised a long time, like souls· bearing
resiliently, on tiny and almost impalpable drops of their essence, the immense
edifice of memory" -Marcel Proust "The Remembrance of Things
Past"(1)
Last week when I was in New York there
was this good smell coming out of this restaurant and right when I smelled it,
the smell brought back memories of this one festival I went to in Japan almost
3 years ago. On another occasion this perfume a girl was wearing brought back
memories of a girlfriend in high school. Of all the senses I would say that
smell is the sense that is best at bringing back memories. When you smell a
certain scent it feels as though you slipped back in time and that you are actually
at that scene again. If it was not for the other senses of your body, you might
really feel as though you are back there again. But why is it that smell has
this ability to instantaneously trigger memories of events, places or people
that you usually would not "think" of?
Despite the tendency of humans to
underestimate the role of smell in our every day lives, for most mammals, smell
is the most important sense. Dogs are probably the most obvious example of
this, it is through the use of the olfactory system that animals are able to
find food, reproduce, and even communicate. While being one of the oldest and
important parts of the brain, our failure to fully realize the importance of
the olfactory system resulted in it being surrounded by numerous questions (2). How does it work?
How do we identify smells? While these are only a few questions out of a whole
list, research has progressed in recent years that we know much more about the
olfactory system than before, but the fact remains that much remains to be
found.
Through research conducted on mice, it
is approximated that humans have 1000 different sensors in their nose (3). While this might
seem like a large amount of sensors, it is not enough considering mice and
humans can identify about 10,000 odors. The mystery surrounding this ratio can
be explained through the unique features of the olfactory system. Odors are
molecular so the method used is different from light or sound that come in
waves (4).
Inside your nose about the level of
your eyes, is a small patch of tissue containing millions of nerve cells. The
odor receptors (sensors) lie on these nerve cells. Each of the receptors
recognizes several odors, and likewise a single odor could be recognized by
several receptors. Thus similar to codes, what happens is that different
combinations of the 1,000 receptors result in our ability to identify 10,000
different odors. Linda Buck, an associate professor at Harvard, makes an
analogy of this quite efficient system to letters being used in different
combinations to make individual words. She goes on to say that this system
'greatly reduces the number of sensors needed to code for the smells" (3).
The process that takes place is quite
complex. After an odor molecule enters the nose and are recognized by the
olfactory sensors, the signals are eventually sent to the olfactory bulb that
is located right above the eyes (3). The signals only go
to two areas in the olfactory bulb, and signals from different sensors are
targeted to different spots that then form a sensory map. From there the
signals reach the olfactory area of the cortex (smell sensory cortex) (5).
An important quality of the olfactory
system is that information travels both to the limbic system and cortex. The
limbic system is the primitive part of the brain that include areas that
control emotions, memory and behavior. In comparison the cortex is the outer
part of the brain that has to do with conscious thought. In addition to these
two areas, information also travels to the taste sensory cortex to create the
sense of flavor (2). Because olfactory
information goes to both the primitive and complex part of the brain it effects
our actions in more ways than we think.
Many wonder how certain smells able to
trigger memories of events taking place several years ago despite the fact that
sensory neurons in the epithelium survive for about only 60 days (1). The answer is that
the neurons in the epithelium actually have successors. As the olfactory
neurons die, new olfactory neurons generated by the layer of stem cells beneath
them, which eventually takes the role of the old neuron as it dies. Linda Buck
points out that the key point to the answer is that "memories survive
because the axons of neurons that express the same receptor always go to the
same place" (1). The memories are
stored in the hippocampus, and through relational memory certain smells trigger
memories.
Another popular question is the reason
behind smell having such a strong role in instantaneously recalling memory.
Despite our belief that sight and hearing are the two most important senses to
our survival, from an evolutionary perspective smell is one of the most
important senses. To recognize food or to detect poison, smell is the sense
that almost all other mammals use. Because of this basic feature yet vital
role, smell is one of the oldest parts of our brain. Trygg Engen, a psychology
professor at Brown University notes that smells serve as "index keys"
to quickly retrieve certain memories in our brain. This primitive yet essential
role is probably why smells trigger memory more than does seeing or hearing.
Professor Engen goes on in attempting
to further explain the relation of odor and memory. His controversial views
basically states that the way we sense odors are all results of "nurture"
and not "nature" (6). He says that
initially all smells are neutral, and that whether a odor is pleasant or
unpleasant has to do with the initial condition in which the smell is
perceived. It follows from this that when we smell odors, it triggers a certain
memory that has to do with that particular odor and thus is decided whether it
is pleasant or unpleasant. Engen's views are controversial because of the lack
of convincing data to back his views up. Although certain points about Engen
seem to make sense, such as how odor serve to trigger memories like index keys,
his views about the "nurture" vs "nature" are a little
harder to understand. If odors are decided by "nurture", it leaves
the question of how so many people have a similar view towards many odors.
There is probably nobody who would say that the smell of rotten food is good.
Yet Engen's views are definitely worth considering because for some odors like
gasoline, some people say it is good while others detest it.
It is said that people can identify
about 10,000 different smells, but have many smells can you name off the top of
your head (3)? In comparison, look
at how many colors there are in a crayon box, or the many varieties of music
existing. This lack of understanding and appreciation of odors is a result of
our over reliance on our eyes and ears, even to the extent that we suppress our
awareness of what our nose tells us. Our underestimation of the role of smell
results in our lack of extensive knowledge concerning many aspects of the
olfactory system. But as Proust stated, smell has such a strong power to
vividly bring back memories, it is definitely more important than we realize.
To a large extent smell is more personal than other senses so it brings back
memories of people, not just places, or things.
WWW Sources
Smell is often our
first response to stimuli. It alerts us to fire before we see flames. It makes us recoil before we
taste rotten food. But
although smell is a basic sense, it's also at the forefront of neurological
research. Scientists are still exploring how, precisely, we pick up odorants,
process them and interpret them as smells. Why are researchers, perfumers,
developers and even government agencies so curious about smell? What makes a
seemingly rudimentary sense so tantalizing?
Smell, like taste, is a chemical sense detected by sensory cells
called chemoreceptors.
When an odorant stimulates the chemoreceptors in the nose that detect smell,
they pass on electricalimpulses to the brain. The brain then interprets patterns in electrical activity as specific
odors and olfactory sensation becomes perception -- something we can recognize
as smell. The only other chemical system that can quickly identify, make sense
of and memorize new molecules is the immune system.
But smell, more so than
any other sense, is also intimately linked to the parts of the brain that
process emotion and associative learning. The olfactory bulb in the brain,
which sorts sensation into perception, is part of the limbic system -- a system that includes the amygdala
and hippocampus, structures vital to our behavior, mood and memory. This link to brain's emotional center makes smell
a fascinating frontier in neuroscience, behavioral science and advertising.
In this article, we'll
explore how humans perceive smell, how it triggers memory and the interesting
(and sometimes unusual) ways to manipulate odor and olfactory perception.
Detection of Odorants
Smell begins when
airborne molecules stimulate olfactory receptorcells. If a substance is somewhat volatile (that is, if
it easily turns into a gas), it will give off molecules, or odorants.
Nonvolatile materials likesteel do not have a smell.
Temperature and
humidity affect odor because they increase molecular volatility. This is why
trash smells stronger in the heat andcars smell musty after rain. A substance's solubility also affects its odor.
Chemicals that dissolve in water or fat are usually intense odorants.
When an air current
sweeps an odorant up through the nostrils, the molecules hit the olfactory epithelium -- the center of olfactory sensation.
The epithelium occupies only about one square inch of the superior portion of
the nasal cavity. Mucus secreted by the olfactory gland coats the epithelium's
surface and helps dissolve odorants.
Olfactory
receptor cells are neurons with knob-shaped tips calleddendrites.
Olfactory hairs that bind with odorants cover the dendrites. When an odorant
stimulates a receptor cell, the cell sends an electrical impulse to the olfactory bulbthrough the axon at its base.
Supporting
cells provide
structure to the olfactory epithelium and help insulate receptor cells. They
also nourish the receptors and detoxify chemicals on the epithelium's surface. Basal stem cells create new olfactory receptors through
cell division. Receptors regenerate monthly -- which is surprising because
mature neurons usually aren't replaced.
While receptor cells
respond to olfactory stimuli and result in the perception of smell, trigeminal nerve fibers in the olfactory epithelium respond to pain. When you smell something caustic like ammonia,
receptor cells pick up odorants while trigeminal nerve fibers account for the sharp sting that makes you
immediately recoil.
But how does odor
actually become smell? In the next section, we'll learn more about olfactory
receptors and odorant patterns.
ANOSMIA
is the inability to
smell. Just as the deaf cannot hear and the blind cannot see, anosmics cannot perceive odor and so
can barely perceive taste. According to the Foundation, sinus disease,
growths in the nasal passage, viral infections and head trauma can all cause the disorder.
Children born with
anosmia often have difficulty recognizing and expressing the disability. Since
anosmics lack the response that alerts us to fire, natural gas leaks and spoiled food, the
Foundation advises installing multiple smoke alarms, switching from gas to electricity and marking all food with expiration dates [source: Foundation].
Olfactory System
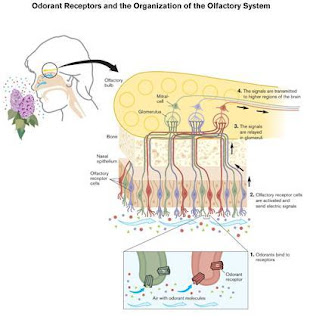
How does the brain recognize, categorize and memorize the huge variety of odors? In 1991,
Richard Axel and Linda Buck published a groundbreaking paper that shed light on
olfactory receptors and how the brain interprets smell. They won the 2004 Nobel Prize in Physiology or Medicine for the paper and their
independent research.
Axel and Buck
discovered a largegene family -- 1,000 genes, or 3 percent of the human
total -- that coded for olfactory receptor types. They found that every
olfactory receptor cell has only one type of receptor. Each receptor type can
detect a small number of related molecules and responds to some with greater
intensity than others. Essentially, the researchers discovered that receptor
cells are extremely specialized to particular odors.
Axel and Buck also
found that each olfactory receptor type sends its electrical impulse to a
particular microregion of the olfactory bulb. The microregion, or glomerulus, that receives the information then passes it on to other parts of the
brain. The brain interprets the "odorant patterns" produced by
activity in the different glomeruli as smell. There are 2,000 glomeruli in the
olfactory bulb -- twice as many microregions as receptor cells -- allowing us
to perceive a multitude of smells.
An illustration of how receptors function in the olfactory system
Another researcher,
however, has challenged the idea that humans have a large number of receptor
types that respond only to a limited number of molecules. Biophysicist Luca
Turin developed the quantum vibration theory in 1996 and suggests that
olfactory receptors actually sense the quantum vibrations of odorants'atoms. While molecular shape still comes into play, Turin purports that the
vibrational frequency of odorants plays a more significant role. He estimates
that humans could perceive an almost infinite number of odors with only about
10 receptors tuned to different frequencies.
Next, we'll learn about
how smells trigger memory and find out how much cognition actually influences
perception.
FOLLOW THE NOSE
The human sense of
smell has long been maligned -- its sensitivity is often unfavorably compared
to that of animals. Smell even came in dead last in a HowStuffWorks battle of
favorite senses.
But researchers at the
University of California at Berkeley have found that humans actually have
sophisticated olfactory capabilities. A group of 32 volunteers were asked to
track scents with their noses across a 10-meter (about 33-foot) trail. The
subjects were blindfolded and wore gloves and earplugs to isolate their senses
of smell. Two-thirds of the volunteers were able to track the scent and,
although they were slower than the tracking dogs, most improved with practice [source: BBC].
Smell and Memory
A smell
can bring on a flood of memories, influence people's moods and even affect
their work performance. Because the olfactory bulb is part of the brain'slimbic system, an area so closely associated with
memory and feeling it's sometimes called the "emotional brain," smell
can call up memories and powerful responses almost instantaneously.
The
olfactory bulb has intimate access to the amygdala, which processes emotion, and thehippocampus, which is responsible for
associative learning. Despite the tight wiring, however, smells would not
trigger memories if it weren't for conditioned responses. When you first smell a new scent,
you link it to an event, a person, a thing or even a moment. Your brain forges
a link between the smell and a memory -- associating the smell of chlorine with summers at the pool or lilies with a funeral. When you
encounter the smell again, the link is already there, ready to elicit a memory
or a mood. Chlorine might call up a specific pool-related memory or simply make
you feel content. Lilies might agitate you without your knowing why. This is
part of the reason why not everyone likes the same smells.
Because
we encounter most new odors in our youth, smells often call up childhood
memories. But we actually begin making associations between smell and emotion
before we're even born. Infants who were exposed to alcohol,
cigarette smoke or garlic in the womb show a preference for the
smells. To them, the smells that might upset other babies seem normal or even
comforting.
In the
next section, we'll find out how some people use smell's ability to trigger
memory.
IS THAT CHEESE OR JUST B.O.?
Researchers
have found that cognition significantly influences the perception of smell. A
psychologist at the University of Oxford labeled an ambiguous Brie-like scent
as either "cheddar cheese" or "body odor." Test subjects
rated the odor higher when it was labeled cheddar cheese. MRIs even showed more activity in the
olfactory region of the brain when subjects believed they were smelling cheese.
Scent Marketing
Advertisers are eager
to cash in on the close link between smell, memory and mood. Real estate agents
have long used scent marketing as a way of putting clients at ease. Sellers set
fresh pie or cookies on countertops to make a house seem comfy and livable. But
because there's a limit to how many pies one agent can bake, companies that
sell aroma-marketing systems are stepping up. Housing developments, hotels,
stores and even car manufacturers are turning to customized scents to help set a mood and
maybe even make an impression.
Scent marketing is the
latest trick to stand out from the visual and auditory barrage that dominates
advertising. These scents, however, are a far cry from the strong smells of
incense and patchouli at the bead store. They're subtle and almost
imperceptible to the unwitting sniffer. Developers use carefully tuned scents
to lure customers into a sense of well-being. Stores that sell shoes or shirts,
items ideally not associated with odor, formulate aromas of ivy or crisp linen.
Some companies even strive to develop a "brand scent," something that
customers will associate with the company as much as a logo.
To learn more about
smell and the other senses, sniff out the links on the next page.
STINK BOMB
While retailers and
developers turn to positive smells for advertising and marketing, the U.S.
Department of Defense has realized the value of bad smells -- really bad
smells. Unlike pepper spray or tear gas, which irritate pain receptors and can cause serious damage, stink
bombs just reek and make unruly crowds disperse in a flash.
The idea of using smell
as a weapon has been around for some time, however. The Office of Strategic
Services for the French Resistance considered using a horrific garbagelike
smell called "Who Me?" against German soldiers in World War II. The only problem? The sulfur that made the scent so pungent had a
nasty habit of escaping on its own and lingering on everything it touched.
The Real Science behind this:
The olfactory
neuroepithelium is located at the upper area of each nasal chamber adjacent to
the cribriform plate, superior nasal septum, and superior-lateral nasal wall.
It is a specialized pseudostratified neuroepithelium containing the primary
olfactory receptors. In neonates, this area is a dense neural sheet, but, in
children and adults, the respiratory and olfactory tissues interdigitate. As
humans age, the number of olfactory neurons steadily decreases. In addition to
the olfactory neurons, the epithelium is composed of supporting cells, Bowman
glands and ducts unique to the olfactory epithelium, and basal cells that allow
for the regeneration of the epithelium.
The sense of smell is
mediated through stimulation of the olfactory receptor cells by volatile
chemicals. To stimulate the olfactory receptors, airborne molecules must pass
through the nasal cavity with relatively turbulent air currents and contact the
receptors. Important determinants of an odor's stimulating effectiveness
include duration, volume, and velocity of a sniff.
Each olfactory
receptor cell is a primary sensory bipolar neuron. The average nasal cavity
contains more than 100 million such neurons. The olfactory neurons are unique
because they are generated throughout life by the underlying basal cells. New
receptor cells are generated approximately every 30-60 days.
Each regenerating
receptor cell extends its axon (cranial nerve I) into the CNS as a first-order
olfactory neuron and forms synapses with target mitral and tufted cells in the
olfactory bulb.
The bipolar olfactory
neurons have a short peripheral process and a long central process. The
peripheral process extends to the mucosal surface to end in an olfactory knob,
which has several immobile cilia forming a dense mat at the mucosal surface.
The cilia express the olfactory receptors that interact with odorants. The
odorant receptors comprise part of a G-protein receptor superfamily associated
with adenylate cyclase. Humans have on the order of 300-400 different active
olfactory receptors, and each neuron expresses only one receptor type.
Receptorlike neurons throughout the epithelium send axons that converge
together within the bundled axons of the fila olfactoria deep to the
epithelium.
These axons project
through the cribriform plate to the ipsilateral olfactory bulb. The olfactory
bulb cells contacted by the olfactory receptor cells include the mitral and
tufted cells, arranged in specialized areas termed glomeruli. The axon
terminals of receptorlike neurons synapse within the same glomeruli, forming an
early topographical odorant map. Therefore, an odor is thought to activate a
set of odorant receptors based on its chemical composition. The corresponding
glomeruli of the olfactory bulbs are in turn activated, creating a unique
pattern of excitation in the olfactory bulb for each odorant.
The glomerular cells
are the primary output neurons of the olfactory bulb. Axons from these cells
travel to the olfactory cortex, which is divided into 5 parts, including (1)
the anterior olfactory nucleus, connecting the 2 olfactory bulbs through the
anterior commissure, (2) the olfactory tubercle, (3) the pyriform cortex, which
is the main olfactory discrimination region, (4) the cortical nucleus of the
amygdala, and (5) the entorhinal area, which projects to the hippocampus.
The olfactory pathway
does not involve a thalamic relay prior to its cortical projections. Relays
from the olfactory tubercle and the pyriform cortex project to other olfactory
cortical regions and to the medial dorsal nucleus of the thalamus and probably
involve the conscious perception of odors.
Conversely, the
cortical nucleus of the amygdala and the entorhinal area are limbic system
components and may be involved in the affective, or hedonic, components of
odors. Regional cerebral blood flow (measured with positron emission
tomography) is significantly increased in the amygdala with introduction of a
highly aversive odorant, and it is associated with subjective ratings of
perceived aversiveness.
The vomeronasal organ
(VNO), or Jacobson organ, is a bilateral membranous structure located within
pits of the anterior nasal septum, deep to the nasal respiratory mucosa and
next to the septal perichondria. Its opening in the nasal vestibule is visible
in 91-97% of adult humans, and it is 2 cm from the nostril at the junction of
the septal cartilage with the bony septum. Unlike lower animals, axons
projecting from the VNO have not been found in postnatal humans.
The VNO is believed
by some to detect external chemical signals termed pheromones or vomeropherins
through neuroendocrine-type cells found within the organ. These signals are not
detected as perceptible smells by the olfactory system and may mediate human
autonomic, psychologic, and endocrine responses.
Free trigeminal nerve
endings, which are stimulated by aversive or pungent stimuli (eg, ammonia),
exist in the nasal mucosa. These are processed via separate pathways from those
in the olfactory system, described above.
Individual taste buds
with multiple receptor cells in each bud mediate taste perception. The taste
buds are modified epithelial cells, not direct neurons as in olfactory
function. These cells have a life span of approximately 10 days and arise
continuously from the underlying basal cell layer in a process of constant
turnover, similar to olfactory receptor cells. Any bud may contain receptors
necessary to identify each different taste.
Afferent nerve
branches making synaptic contact with receptor cells penetrate the base of the
taste bud. Taste buds occupy papillae, which are projections embedded in the
tongue epithelium. A single nerve fiber innervates multiple taste papillae, and
the nerve contact exerts trophic influences on the epithelium.
The specificity of
the gustatory receptor cells is determined by the epithelium in which it
resides, not by the particular nerve innervating the bud. A single fiber in the
chorda tympani may respond to multiple types of tastes, some tastes more than
others. This ability of single nerve fibers to respond to multiple types of
stimuli is referred to as broad tuning, and it is shared by the olfactory
system.
Lingual papillae have
the following 4 forms, each occupying different areas of the tongue:
·
Fungiform papillae are located in the
anterior two thirds of the tongue. People have an average of 33 fungiform
papillae with approximately 114 buds per papilla. Innervation is through
cranial nerve (CN) VII via the chorda tympani.
·
Circumvallate papillae are located in
the posterior two thirds of the tongue, consisting of 8-12 papillae,
approximately 250 buds each, for an average of 3000 total buds. Cranial nerve
IX innervates these, along with the entire posterior one third of the tongue.
·
Foliate papillae reside in folds and
clefts at the lateral borders of the tongue, with approximately 1280 buds.
Cranial nerve IX innervates these buds.
·
Filiform papillae have no taste buds.
Other locations of
taste buds include the following:
·
Soft palate - Innervated by CN VII via
the greater superficial petrosal nerve
·
Epiglottis and larynx - Supplied by the
superior laryngeal branch of CN X
·
Pharynx - Supplied by branches from CN
IX and CN X
Free trigeminal nerve
endings exist on the tongue; these detect strong, often displeasing or
irritating sensations in the oral cavity.
Five different taste
qualities–salty, sweet, sour, bitter, and umami (monosodium glutamate/ 5'
nucleotide)–have been identified. They can be detected in all regions of the
tongue, but certain areas of the tongue have lower thresholds for each quality.
Sweetness is most readily detected at the tip of the tongue, whereas salty
taste receptors focus on the anterolateral borders. Sour tastes are best
perceived along the lateral border, and bitter sensations are tasted most in
the posterior one third. Another proposed taste quality is chalky (calcium
salts).
Etiology of Smell and Taste Disorders
Olfactory dysfunction
Disturbances
in olfaction can result from pathologic processes at any level along the
olfactory pathway. They can be thought of similarly to otologic dysfunctions as
conductive or sensorineural defects.
In
conductive (ie, transport) defects, transmission of an odorant stimulus to the
olfactory neuroepithelium is disrupted. Sensorineural defects involve the more
central neural structures. Overall, the most common causes of primary olfactory
deficits are nasal and/or sinus disease, prior viral upper respiratory infections (URIs), and head trauma.
·
Conductive
defects
o
Inflammatory
processes cause a large portion of olfactory defects. These may include
rhinitis of various types, including allergic, acute, or toxic (eg, cocaine
use). Chronic rhinosinusitis causes progressive mucosal disease and often leads
to decreased olfactory function despite aggressive allergic, medical, and
surgical intervention.
o
Masses
may block the nasal cavity, preventing the flow of odorants to the olfactory
epithelium. These include nasal polyps (most common), inverting papilloma, and
any malignancy.
o
Developmental
abnormalities (eg, encephaloceles, dermoid cysts) also may cause obstruction.
o
Patients
with laryngectomies or tracheotomies experience hyposmia because of a reduced
or absent nasal airflow. Children with tracheotomies who are cannulated very
young and for a long period may have a continued problem with olfaction even
after decannulation because of a lack of early stimulation of the olfactory
system.
·
Central/sensorineural
defects
o
Infectious
and Inflammatory processes contribute to central defects in olfaction and in
transmission. These include viral infections (which may damage the
neuroepithelium), sarcoidosis (affecting neural structures), Wegener
granulomatosis, and multiple sclerosis.
o
Congenital
causes may be associated with neural losses. Kallman syndrome is one type of congenital
smell loss and is due to failed olfactory structure ontogenesis and
hypogonadotropic hypogonadism. One study found the VNO to be absent in patients
with Kallman syndrome.
o
Endocrine
disturbances (eg, hypothyroidism, hypoadrenalism, diabetes mellitus) may affect
olfactory function.
o
Head
trauma, brain surgery, or subarachnoid hemorrhage may stretch, damage, or
transect the delicate fila olfactoria or damage brain parenchyma and result in
anosmia.[2]
o
Toxicity
of systemic or inhaled drugs (eg, aminoglycosides, formaldehyde) can contribute
to olfactory dysfunction. Many other medications and compounds may alter smell
sensitivity, including alcohol, nicotine, organic solvents, and direct
application of zinc salts.
o
Over-the-counter
zinc nasal sprays have been implicated in the cause of smell loss. On June 16,
2009, the US Food and Drug Administration (FDA) issued a public health advisory
and notified consumers and health care providers to discontinue use of
intranasal zinc products. The intranasal zinc products (Zicam Nasal Gel/Nasal
Swab products by Matrixx Initiatives) are herbal cold remedies that claim to
reduce the duration and severity of cold symptoms and are sold without a
prescription. The FDA received more than 130 reports of anosmia (inability to
detect odors) associated with intranasal zinc. Many of the reports described
the loss of smell with the first dose.[3]
o
The
number of fibers in the olfactory bulb decreases throughout one's lifetime. In
one study the average loss in human mitral cells was 520 cells per year with a
reduction in bulb volume of 0.19 mm3.[4] These olfactory bulb losses may be
secondary to sensory cell loss in the olfactory mucosa and/or general decline
in the regenerative process from stem cells in the subventricular zone.
o
Degenerative
processes of the central nervous system (eg, Parkinson disease, Alzheimer
disease, normal aging) have been found to cause hyposmia. In the case of
Alzheimer disease, olfactory loss can be the first symptom of the disease
process. The sense of smell, more than taste, is impaired with aging, with a
noticeable average decline in function during the seventh decade of life.
Once
thought to be mostly a conductive defect through mucosal edema and polyp
formation, chronic rhinosinusitis also appears to disrupt the neuroepithelium
with irreversible loss of olfactory receptors through upregulated apoptosis.
Gustatory dysfunction
Much of
what is perceived as a taste defect is truly a primary defect in olfaction,
which alters flavor. The components that comprise the sensation of flavor
include the food's smell, taste, texture, and temperature. Each of these
sensory modalities is stimulated independently to produce a distinct flavor
when food enters the mouth.
Taste may
be enhanced by tongue movements, which increase the distribution of the
substance over a greater number of taste buds. Adaptation in taste perception
exerts a greater influence than in other sensory modalities.
Other than
smell dysfunction, the most frequent causes of taste dysfunction are prior URI,
head injury, and idiopathic causes, but many other causes can be responsible.
·
Lesions
at any site from the mucosa, taste buds, unmyelinated nerves, or cranial nerves
to the brain stem may impair gustation.
·
Oral
cavity and mucosal disorders including oral infections, inflammation, and
radiation-induced mucositis can impair taste sensation. The site of injury with
radiotherapy is probably the microvilli of the taste buds, not the taste buds
themselves, since taste buds are thought to be radioresistant.
·
Poor
oral hygiene is a leading cause of hypogeusia and cacogeusia. Viral, bacterial,
fungal, and parasitic infections may lead to taste disturbances because of
secondary taste bud involvement.
·
Normal
aging produces taste loss due to changes in taste cell membranes involving
altered function of ion channels and receptors rather than taste bud loss.
·
Malignancies
of the head and neck, as well as of other sites, are associated with decreased
appetite and inability to appreciate flavors.
·
Use
of dentures or other palatal prostheses may impair sour and bitter perception,
and tongue brushing has been shown to decrease taste acuity.
·
Surgical
manipulation may alter taste permanently or temporarily.
o
Resection
of the tongue and/or portions of the oral cavity most commonly for reasons of
malignancy decreases number of taste buds.
o
Radiation
and chemotherapy damages taste receptors and decreases salivary flow altering
taste perception.
o
In
otologic surgery, stretching or transection of the chorda tympani nerve may
result in temporary dysgeusia. Bilateral injury still may not result in
permanent taste dysfunction because of the alternate innervation through the
otic ganglion to the geniculate ganglion via the greater superficial petrosal
nerve.
·
Nutritional
deficiencies are involved in taste aberrations. Decreased zinc, copper, and
nickel levels can correlate with taste alterations. Nutritional deficiencies
may be caused by anorexia, malabsorption, and/or increased urinary losses.
·
Endocrine
disorders also are involved in taste and olfactory disorders. Diabetes
mellitus, hypogonadism, and pseudohypoparathyroidism may decrease taste
sensation, while hypothyroidism and adrenal cortical insufficiency may increase
taste sensitivity. Hormonal fluctuations in menstruation and pregnancy also
influence taste.
·
Heredity
is involved in some aspects of gustation. The ability to taste phenylthiourea
(bitter) and other compounds with an –N-C= group is an autosomal dominant
trait. Studies have shown that phenylthiourea tasters detect saccharin,
potassium chloride (KCl), and caffeine as more bitter. Type I familial
dysautonomia (ie, Riley-Day syndrome) causes severe hypogeusia or ageusia
because of the absence of taste bud development.
·
Direct
nerve or CNS damage, as in multiple sclerosis, facial paralysis, and thalamic
or uncal lesions, can decrease taste perception.
·
Many
other diseases can affect gustation (eg, lichen planus, aglycogeusia, Sjögren
syndrome, renal failure with uremia and dialysis, erythema multiforme,
geographic tongue, cirrhosis).
Diagnosis of Smell and Taste
Disorders
The first
step in diagnosing any deficit of taste and smell is obtaining a thorough
history and physical examination. Give attention to any antecedent URI, nasal
or sinus pathology, history of trauma, other medical problems, and medications
taken.
Order sinus
CT scans if the history and examination are not consistent with a common
pattern (gradually progressing olfactory loss in a 38-year-old male).
Generally, olfactory loss in the absence of CNS symptoms or an abnormal
neurologic examination is highly unlikely to be associated with an intracranial
mass such as a meningioma. However, an MRI of the brain is often recommended
when the history is not straightforward or a secondary neurologic symptom or
sign is obtained. Although a standard laboratory panel is not recommended, tests
to evaluate for allergy, diabetes mellitus, thyroid functions, renal and liver
function, endocrine function, and nutritional deficiencies may be obtained
based on history and the physical examination. Olfactory epithelium biopsy is
used primarily as a research technique.
Clinical measurement of olfaction
Quantitative
measurement of smell and taste dysfunctions is most important when chemosensory
dysfunction is the primary symptom. The major goal of sensory testing is to
assess the degree of chemosensory dysfunction.
Clinical
testing can be time consuming and difficult to perform precisely, but some
commercially available tests attempt to simplify and standardize these efforts.
Tests of
olfactory function that evaluate threshold of odor detection and odor
identification have been developed that can provide a reliable measure of
olfactory ability. These tests include butanol threshold test, the University
of Pennsylvania Smell Identification Test (UPSIT), and the Sniffin' Sticks
test. Another test, the olfactory-evoked response, has been used in research
centers along with odor identification tests to evaluate aberrant olfaction
with relation to neurologic disease.
·
Butanol
threshold test
o
The
butanol threshold test involves a forced-choice test using an aqueous
concentration of butyl alcohol in one sniff bottle and water in the other. The
patient is asked to identify the bottle containing the odorant, with each
nostril tested separately.
o
After
each incorrect response, the concentration of butanol is increased by a factor
of 3 until the patient either achieves 5 correct responses or fails to
correctly identify the bottle with 4% butanol.
o
The
detection threshold is recorded as the concentration at which the patient
correctly identifies the butanol on 5 consecutive trials. The scoring relates
the patient's threshold to a normal subject population
·
University
of Pennsylvania Smell Identification Test
o
The
UPSIT involves 40 microencapsulated odors in a scratch-and-sniff format, with 4
response alternatives accompanying each odor. The patient takes the test alone,
with instructions to guess if not able to identify the item.
o
Anosmic
patients tend to score at or near chance (10/40 correct). The scores are
compared against sex- and age-related norms, and the results are analyzed. This
test has excellent test-retest reliability.
o
A
chart is available relating scores to varying patient populations, including
patients with multiple sclerosis, with Korsakoff syndrome, and those feigning
anosmia. Those in the latter group tend to score much lower on the test than
expected by chance.
·
Cross-Cultural
Smell Identification Test
o
A
variant of the UPSIT, which can be given in 5 minutes, was proposed for a quick
measure of olfactory function. The 12-item Cross-Cultural Smell Identification
Test (CC-SIT) was developed using input on the familiarity of odors in several
countries, including China, Colombia, France, Germany, Italy, Japan, Russia,
and Sweden.
o
The
odorants chosen include banana, chocolate, cinnamon, gasoline, lemon, onion,
paint thinner, pineapple, rose, soap, smoke, and turpentine. Representatives
from each country identified these odorants most consistently.
o
This
test is an excellent alternative for measuring olfactory function in the
clinical setting, especially when time is limited, since it is rapid and
reliable.
o
The
disadvantage of this test is that its brevity limits its sensitivity in
detecting subtle changes in olfactory function.
·
Sniffin'
Sticks
o
Uses
a series of reusable penlike odor-dispensing devices
o
Tests
odor threshold through a single staircase method, odor discrimination with
forced choice among 3 of 16 different common odorants, and odor identification
with multiple forced choice from 4 verbal items.
o
A
composite score is calculated from a composite of all 3 scores to provide an
overall evaluation of olfactory function.
·
Olfactory-evoked
response
o
To
standardize the patient reaction to eye movements, electroencephalogram (EEG)
electrodes and an electrooculogram measure olfactory-evoked potentials. A
visual tracking task is performed to ensure constant alertness to the task, and
headphones playing white noise are worn to mask auditory clues.
o
Either
carbon dioxide (no odor but a trigeminal stimulant) or hydrogen sulfide is
delivered via an olfactometer to the nose in a constantly flowing air stream.
N1 is the first negative peak measured, and P2 is the second positive trough.
Latencies are measured to these 2 values.
o
In
patients with neurologic disease, the UPSIT revealed abnormality more
frequently than olfactory-evoked responses.
For
clinical olfactory function testing, the authors' experience is that the
self-administered UPSIT test allows for practical use during a busy clinical
practice. However, in the absence of the olfactory tests described above, a
simple screening test using a common alcohol pad can be used. The envelope is
opened at one end and presented to the patient. With the patient's eyes closed,
the pad is then positioned at the level of the umbilicus and slowly brought
closer to the nose. The patient is instructed to notify the tester when the
alcohol is again detected. The distance of the pad from the nose correlates
with the patient's olfactory ability, with a distance of less than 20 cm
indicating hyposmia.
Clinical measurement of taste
Evaluation
of taste disorders is not as well developed as that of olfaction. It involves
measurement of detection or recognition thresholds. No comparable approach to
odor identification tests is available because only 5 basic taste sensations
exist and only 4 of these (sweet, salty, bitter, and sour) are tested.
Salivary
adaptation and size of the tongue area stimulated influence the threshold
assessment. Thus, these tests are extremely variable. Changes in threshold
detection do not necessarily indicate correlation to changes in suprathreshold
taste intensity. Testing of the taste thresholds alone does not provide a full
picture of the level of gustatory function or dysfunction. For example, a
patient after radiation therapy may recover recognition thresholds for the 4
taste qualities, but the magnitude of the perceived tastes still may be quite
depressed.
·
Magnitude
matching
o
Suprathreshold
testing involves assessment of the patient's perceptions of taste intensities
at levels above threshold. One method of measuring this quality is with a
psychophysical procedure known as magnitude matching.
o
Other
tests of suprathreshold tastes have involved assigning numbers to their
sensations, but no direct comparison across individuals can be made. Specific
numbers, such as 10 or 100, do not have any intrinsic psychologic value.
o
Conversely,
magnitude matching makes use of one sensory modality that is presumed to be
normal (in this case, hearing) in comparison to a deficiency in another sensory
modality (taste) by using the following procedure:
§
Several
concentrations of sodium chloride, sucrose, citric acid, and quinine
hydrochloric acid, along with several loudness levels of a 1000-Hz tone, are
provided for the magnitude matching task.
§
The
patient sips each solution and expectorates, and the tones are presented via
headphones. The patient provides estimates of perceived magnitude for each
stimulus.
§
The
results are scaled in relation to loudness functions to reveal abnormalities of
taste as depressed psychophysical functions. In other words, patients with
hypogeusia associate stronger taste concentrations with weaker tones than
normal patients.
§
The
major limitations of this testing modality are its dependence on normal hearing
and its complicated design, which takes a significant amount of time to administer
and analyze.
·
Spatial
test
o
Taste
function in the various areas of the tongue and oral cavity can be measured
using a spatial test. Because the gustatory system is multiply innervated,
damage to one of the 3 major nerves (ie, chorda tympani, glossopharyngeal,
greater superficial petrosal) or their ganglia may cause a disturbance of taste
that can be evaluated only by testing the anatomic areas supplied by those
nerves.
o
To
test these areas, 4 standardized sizes of filter paper are soaked with strong
concentrations of the 4 basic tastes. The papers are randomly placed on the 4
quadrants of the tongue and on both sides of the soft palate. Patients then
identify the quality of the taste and rate its intensity using the same scale
as in whole mouth assessment.
Treatment of olfactory dysfunction
Any treatment of
olfactory disorders must first treat the specific causative abnormality if it
has been identified from diagnostic tests, history, and physical examination.
·
Local nasal and/or sinus conditions should
be optimally managed with saline lavage, decongestants, antihistamines,
antibiotics, and/or nasal and systemic steroids, if applicable. Polyps and
sinus disease that are resistant to medical management should be surgically
addressed to remove the conductive defect. Care must be exercised during
surgery to avoid damage to the olfactory regions.
·
Aggressive treatment of these
disorders, if present, provides a good chance of improvement. In general,
conductive olfactory losses are the most amenable to treatment.
·
A few of the sensorineural olfactory
defects also have specific treatments, but these are fewer and have less chance
of success. Generally, viral processes that damage the olfactory
neuroepithelium, sarcoidosis, and multiple sclerosis do not have specific
remedies; however, steroids may be administered in an attempt to limit the
inflammation.
·
Endocrine disturbances may be addressed
by administration of the deficient hormone, as with hypothyroidism. Control of
diabetes mellitus may slow neural degeneration of the olfactory system.
·
Idiopathic cases of olfactory loss are
not amenable to specific treatment, although some unproven remedies have been
attempted. The best known of these is zinc sulfate. It has not been proven
beneficial and is generally regarded as ineffective.
·
Other unproven remedies include
pharmacologic doses of vitamins, topical steroids, and tricyclic
antidepressants (for their effect on CSF catecholamines). Oral steroids, once
thought to benefit only those with polyp disease, have recently shown to
improve olfactory function in patients with sensorineural defects as well as
conductive disorders.
·
A viral URI can cause extensive
scarring and replacement of the olfactory neuroepithelium with respiratory
epithelium, but studies suggest that stem cells remain, allowing for potential
regeneration of the olfactory epithelium. Recovery of smell in these cases can
take weeks to months and, in some instances, may never occur. Unfortunately,
besides the possibility of oral steroids as mentioned above, no proven therapy
exists to improve function in these patients.
·
Eliminating toxins (eg, cigarette
smoke, airborne pollutants) may help.
·
Overall, the patient with olfactory
disorders needs reassurance that these generally are not life-threatening problems
and that many other individuals experience them. In some patients, psychiatric
evaluation and treatment may be warranted. Most importantly, the physician is
responsible for warning the patient with olfactory disorders of the hazards
associated with the inability to smell odors such as smoke, natural gas leaks,
and spoiled food. Smoke detectors, as well as natural gas and propane gas
detectors, are commercially available to help eliminate such risks.
Treatment of gustatory dysfunction
As with olfactory
problems, direct initial treatment of gustatory dysfunction toward the
causative abnormality, if possible.
·
Address any nasal pathology causing
decreased olfaction and thus affecting taste.
·
Treat mucosal disorders (eg,
infections, inflammations).
·
Treat oral candidiasis and other local
factors, and replete any vitamin deficiency that may cause glossitis.
·
Aid patients in eliminating local
irritants (eg, mouthwashes, ill-fitting dentures)
·
In mucositis or dry mouth as a result
of radiation therapy, artificial saliva or salivary stimulants and local
anti-inflammatory medications may improve some taste dysfunction.
·
Correcting endocrine disorders with the
appropriate hormone replacement may improve the taste disorder.
·
Consider eliminating a medication
suspected of causing dysgeusia unless the medication is crucial in treating
another medical problem and cannot be substituted.
·
In the case of familial dysautonomia,
in which patients have a complete lack of lingual taste buds, subcutaneous
administration of methacholine has been reported to normalize previously
elevated taste thresholds for all taste qualities. The cholinergic mechanism is
probably related to taste transduction via free nerve endings because these
patients have no taste receptors.
·
Some gustatory deficits are untreatable
(eg, some cases of nerve or CNS damage, end-stage diabetic neuropathy, multiple
sclerosis). Certain mechanical aids exist to enable the patient to make use of
whatever taste function is left.
·
Advise patients that chewing food well
increases the release of the tastant and increases saliva production to further
distribute the chemicals. Switching foods during the meal decreases the
phenomenon of adaptation and can improve detection of the tastes.
·
Finally, for patients who are anosmic
or hyposmic (including many elderly people), simulated odors are available to
use while cooking to augment the sensation of flavor. A drawback of these
simulated odors is that, to normosmic people, the smell is quite pungent. Thus,
these odors cannot be used in mixed groups of anosmic and normosmic
individuals.
Summary
Smell and
taste disorders traditionally have been overlooked in most aspects of medical
practice because these specialized senses often are not considered critical to
life. However, they affect everyday enjoyment of food, and they impair
detection of the potentially dangerous smells of smoke or spoiled food.
Anxiety and
depression, as well as anorexia and nutritional deficiencies, may result from
taste and smell disorders. Many causes of smell and taste disorders exist, and
the modalities of treatment begin with treating the specific deficit, if
possible.
Unfortunately,
much about the diagnosis and treatment of taste and smell dysfunction remains
to be discovered. Most taste defects are truly alterations in perception of
flavor due to smell defects, and they should be treated accordingly.
Some
standardized tests, such as the butanol threshold, odor identification,
Sniffin' Sticks, UPSIT, and olfactory-evoked potentials, can help diagnose and
measure olfactory dysfunction; however, diagnosis remains an imprecise science.
Measurement of gustatory disturbances is even less precise and more difficult.
Reassurance
is one of the most important aspects of treatment in these disorders because
cures are often difficult to obtain and may take weeks, months, or years.